Membrane Electrolysis (ME)
The production of metallic powders through membrane electrolysis involves the electrolytic reduction of metal ions in an electrolyte solution. Metal ions are reduced at the cathode (negative electrode) to form metallic powders. When an external voltage is applied to the electrodes, metal ions in the electrolyte are attracted to the cathode. These metal atoms then aggregate and convert into metallic powders on the surface of the cathode. The metallic powders that form on the cathode are collected and subsequently prepared through methods such as washing and drying.
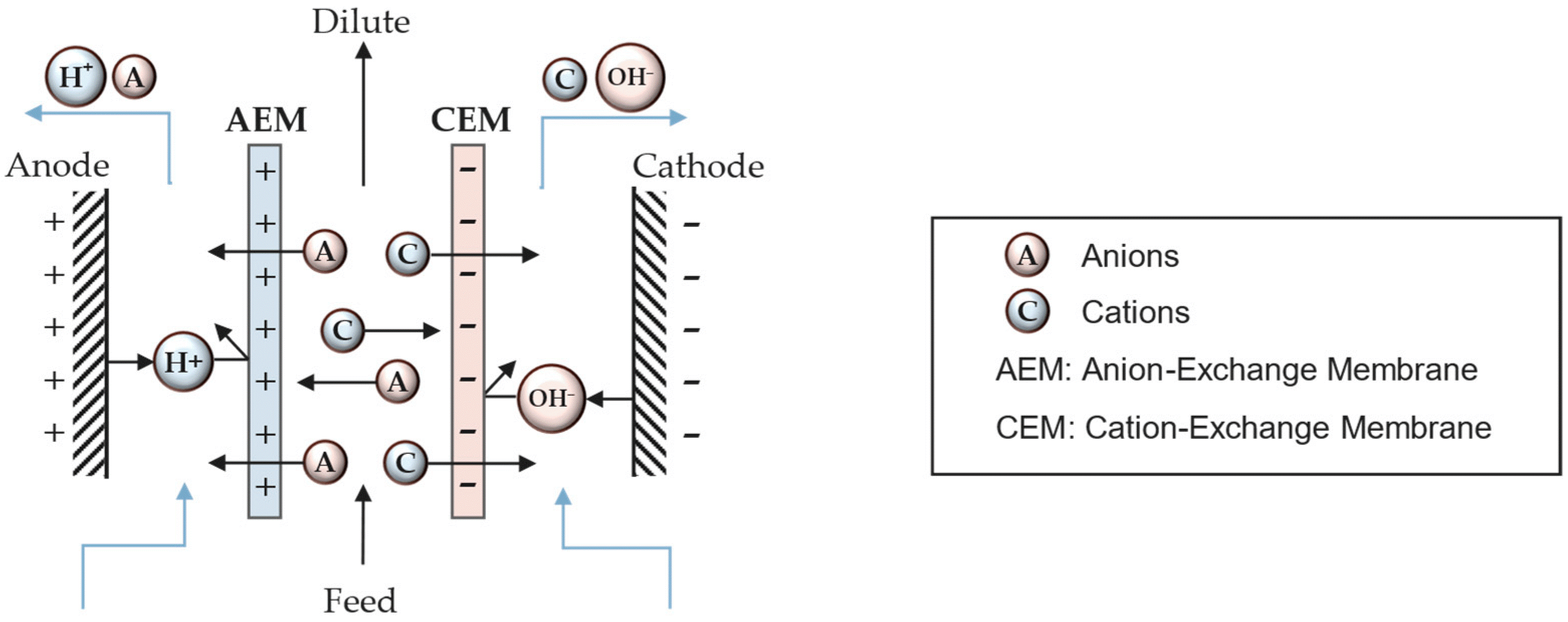
Various factors such as current density, electrolyte composition, temperature, pH, and electrode materials can influence the characteristics of the produced metallic powders. Controlling these parameters is crucial for producing suitable powders. In fact, by managing the aforementioned parameters, metal oxides and hydroxides can also be produced.
The use of the membrane electrolysis process makes our products unique. Our specialized products include:
– Nano and Micro Copper Powder (smaller than 3 micrometers): Suitable for conductive ink applications with exceptional electrical conductivity.
– Nano Zinc Oxide Powder (ZnO): Exhibiting antibacterial properties suitable for health and hygiene applications.
– Nano Aluminum Hydroxide Powder (needle-like): Offering thermal stability suitable for catalytic applications and refractory production.
– Nickel Hydroxide, Core-Shell Nano Nickel Hydroxide/Nickel.
Material | Electrical Explosion of Wire (in Liquid Environment) | Electrical Explosion of Wire (in Gas Environment) | Membrane Electrolysis |
Copper | ✔ | ✔ | ✔ |
Nickle hydroxide | ✔ | ✔ | |
Ni hydroxide-Ni core-shell | ✔ | ||
Molybdenum Oxide | ✔ | ||
Tungsten Oxide | ✔ | ||
Tungsten | ✔ | ||
Gold | ✔ | ✔ | |
Platin | ✔ | ✔ | |
Silver | ✔ | ✔ | ✔ |
Aluminum | ✔ | ||
Boehmite | ✔ | ✔ | |
Zinc oxide | ✔ | ✔ | ✔ |
Tantalum Oxide | ✔ | ||
Metal Alloys | ✔ | ✔ |
